Occluder testing - Cardiovascular implants
Questmed GmbH is a test laboratory for occluder and closure device testing of cardiovascular implants.We are testing cardiovascular implants like occluder, stents and vena cava filters for fatigue and durability and other mechanical loads in accordance with ISO 22679, ASTM F3211, ISO 25539-1, ISO 25539-2 and ISO 25539-3.
- Fatigue testing
- Durability testing
- Corrosion characterization
- Visual inspection
- Dimensional verification
For fatigue and durability testing with axial and hydrodynamic loads we use our accredited test procedure.
We are the first and only test lab with an accreditation for ISO 22679 Occluder - Durability under axial and hydrodynamic load testing.

We have experience since 2011 for testing all kinds of occluders, for instance
- patent foramen ovale occluder (PFO),
- atrial septal occluder (ASD),
- patent ductus arteriosus occluder (PDA),
- ventricular septal occluder (VSD),
- paravalvular leak occluder (PVL),
- left atrial appendage occluder (LAA),
- atrial flow regulator (AFR / IASD),
- cardiac patches or strips.
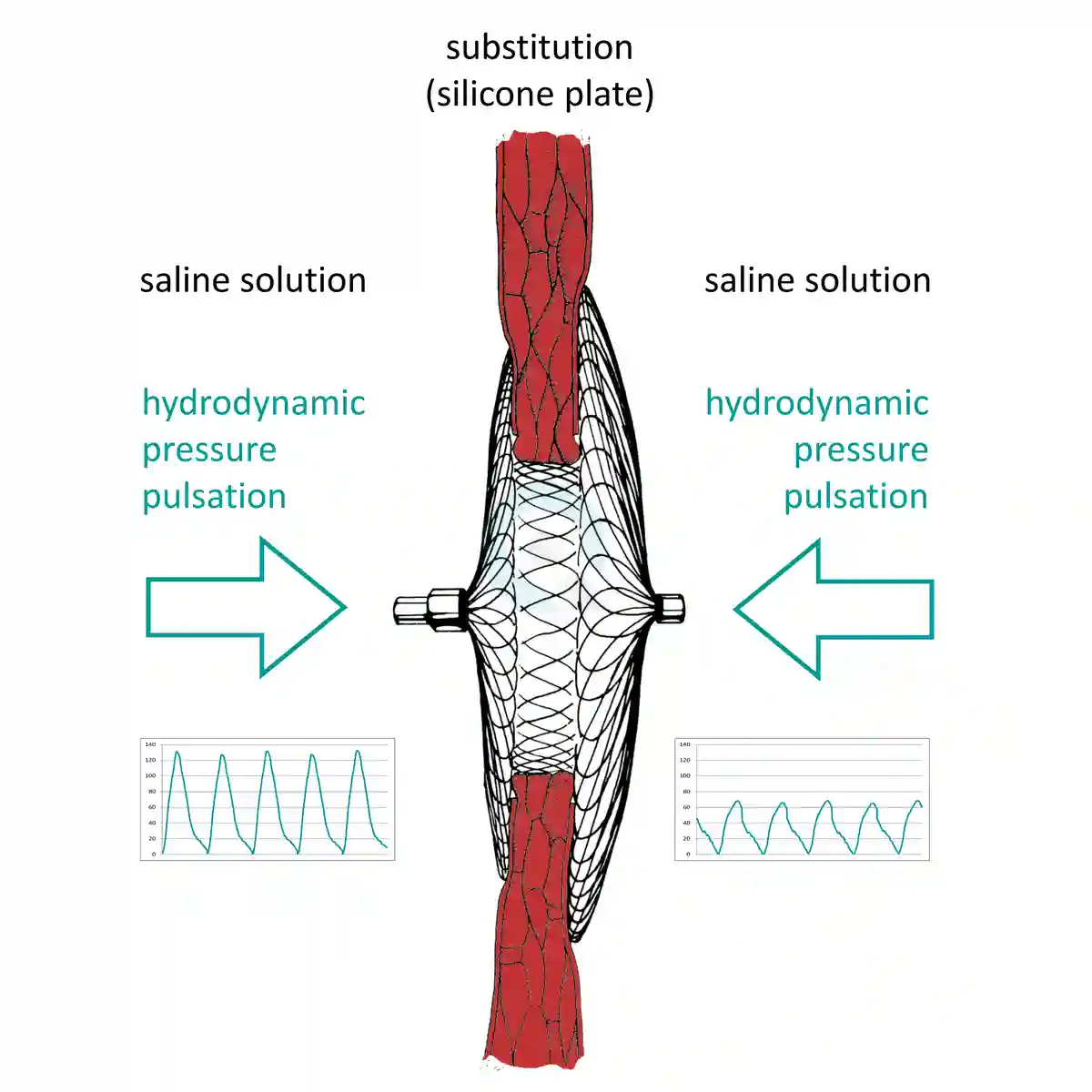
Differential Hydrodynamic Pressure Test
The test should run according to ISO 22679 Annex H.3 "Durability of the occluder implant".Fatigue and durability tests run with 1.2 Hz up to 90 Hz at 37 °C tempered 0.9 % saline solution.
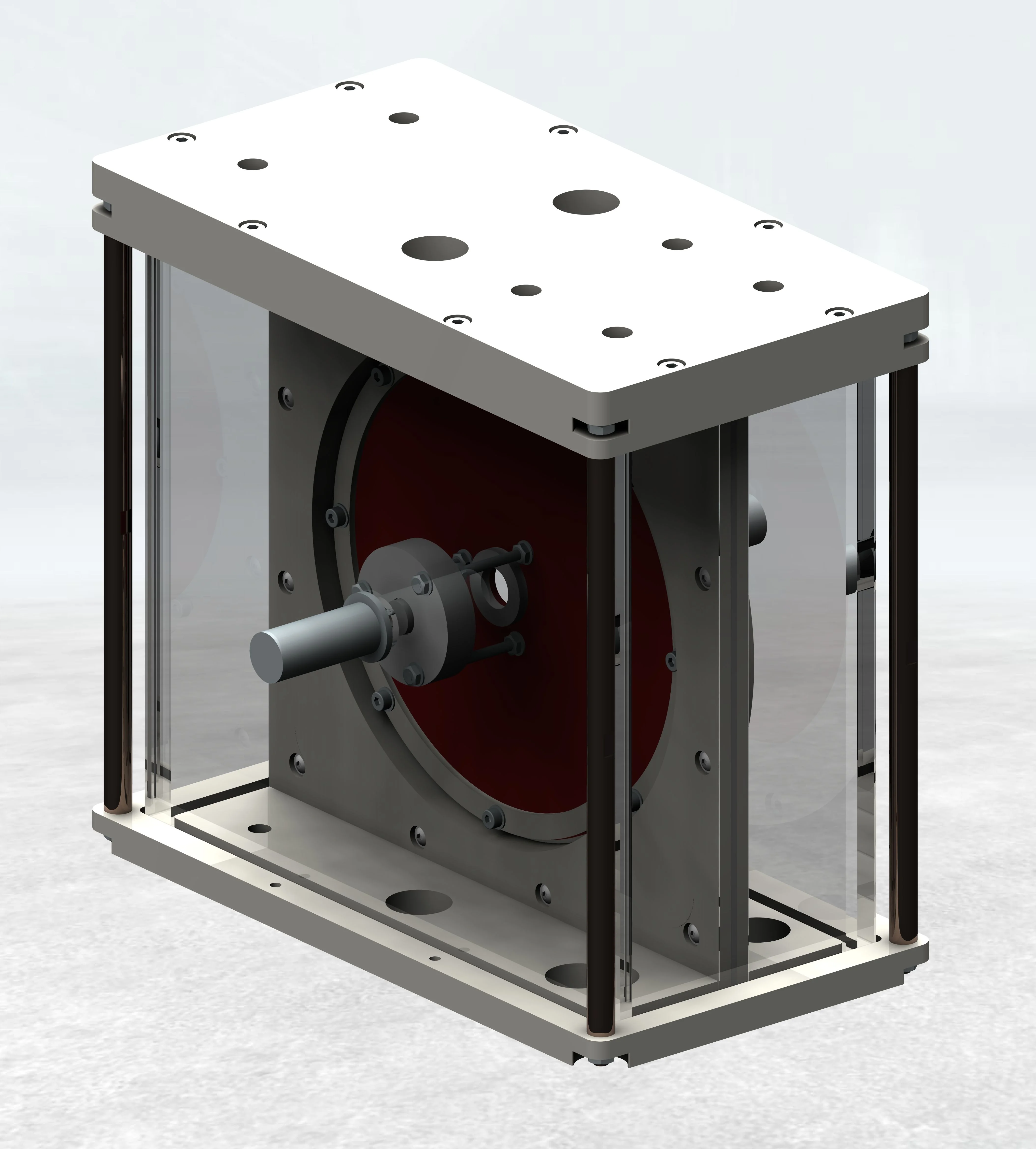
Our multidevice cardiac implant test systems for occluder are available for 1 to 30 Samples.
Occluder are mounted using flexible adapter up to 60 mm in height and 60 mm in diameter.
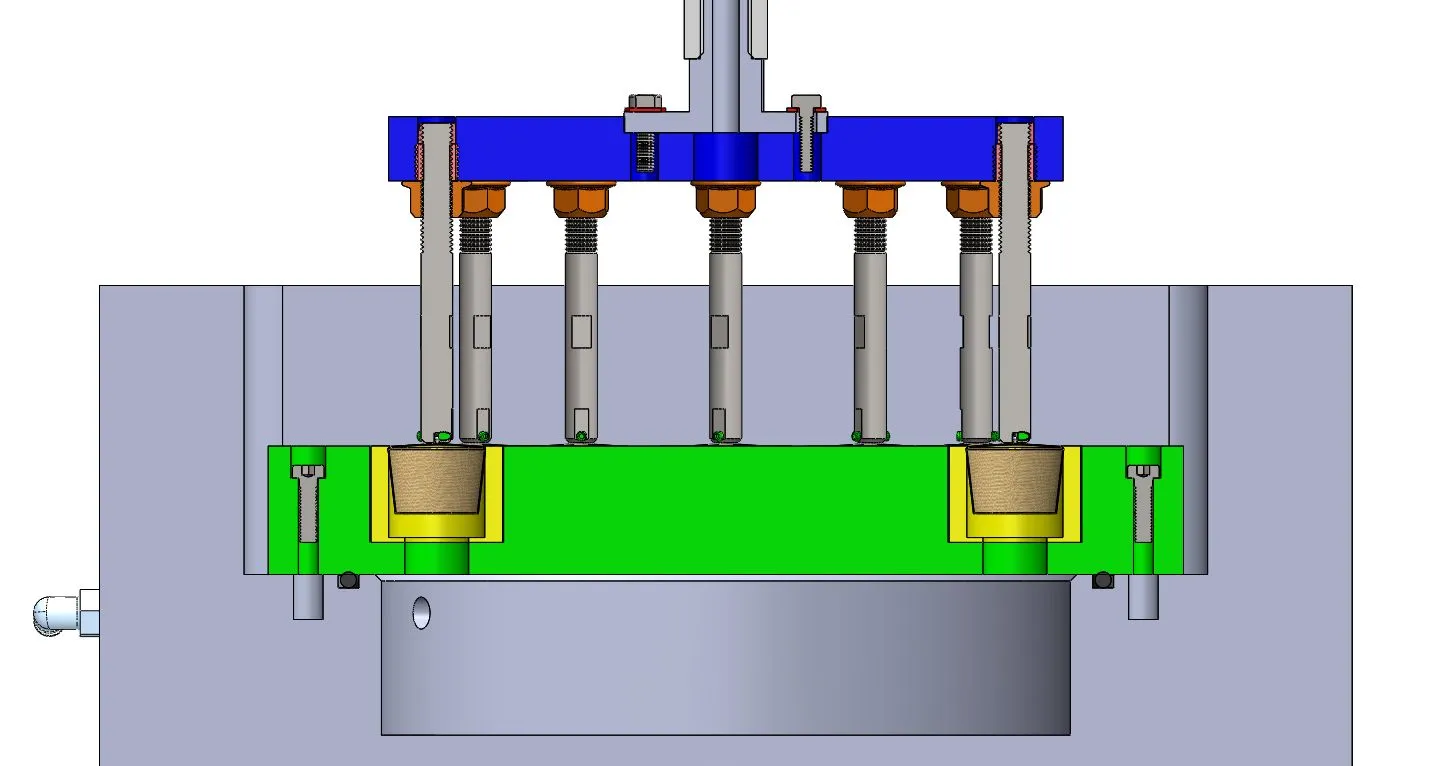
High cycle fatigue and durability test demonstrates the occluder´s ability to withstand physiological stresses during long term implantation.
We perform pulsatile fatigue and durability test for 400 million cycles (customer specification of 10 million cycles is possible).
Our test systems provides highspeed pressure tests with differential hydrodynamic pressures to the occluder with highspeed data recording to test the performance of Occluders using the Questmed OTV test system.
Pulsatile flows simulate the intended deployment location for occluder and closure devices.
Steady flow will also be performed to better understand the fundamental operation of devices.
Tests include:
- fatigue testing
- durability testing
- short-term and long-term pressurization
- interim visual inspections without removing the test samples
- stroboscope Visual Inspection
- metal ion analysis like nickel and titanium
Differential Pressure Pulsation for Fatigue and Durability Testing sorted by type of Occluders
- Type - Differential Pressure pulsation (ISO 22679 Annex O)
- PFO - diff 14 mmHg
- ASD - diff 14 mmHg
- PDA - diff 110 mmHg
- VSD - diff 110 mmHg
- PVL - diff 119 mmHg
- LAA - diff 27 mmHg (persistent atrial fibrillation)
- LAA - diff 18 mmHg (contraindication atrial fibrillation)
- AFR - diff 30 mmHg (interatrial shunt device IASD)
- AAA - diff 20 mmHg
Sample Size Calculation
Sample size is not defined in the ISO 22679 standard for occluder fatigue and durability testing.
29 samples have to be tested, for example, when all test samples have to fulfill requirements and 95% confidence and 90% reliability is used. Calculation is based on ASTM F3172 table 3.
Axial Fatigue and Durability Testing of Occluders
Axial tests run from 10 Hz up to 90 Hz in 37 °C tempered 0.9 % saline ringer's solution.Our multidevice cardiac implant test system for occluders is available for 12 Samples.
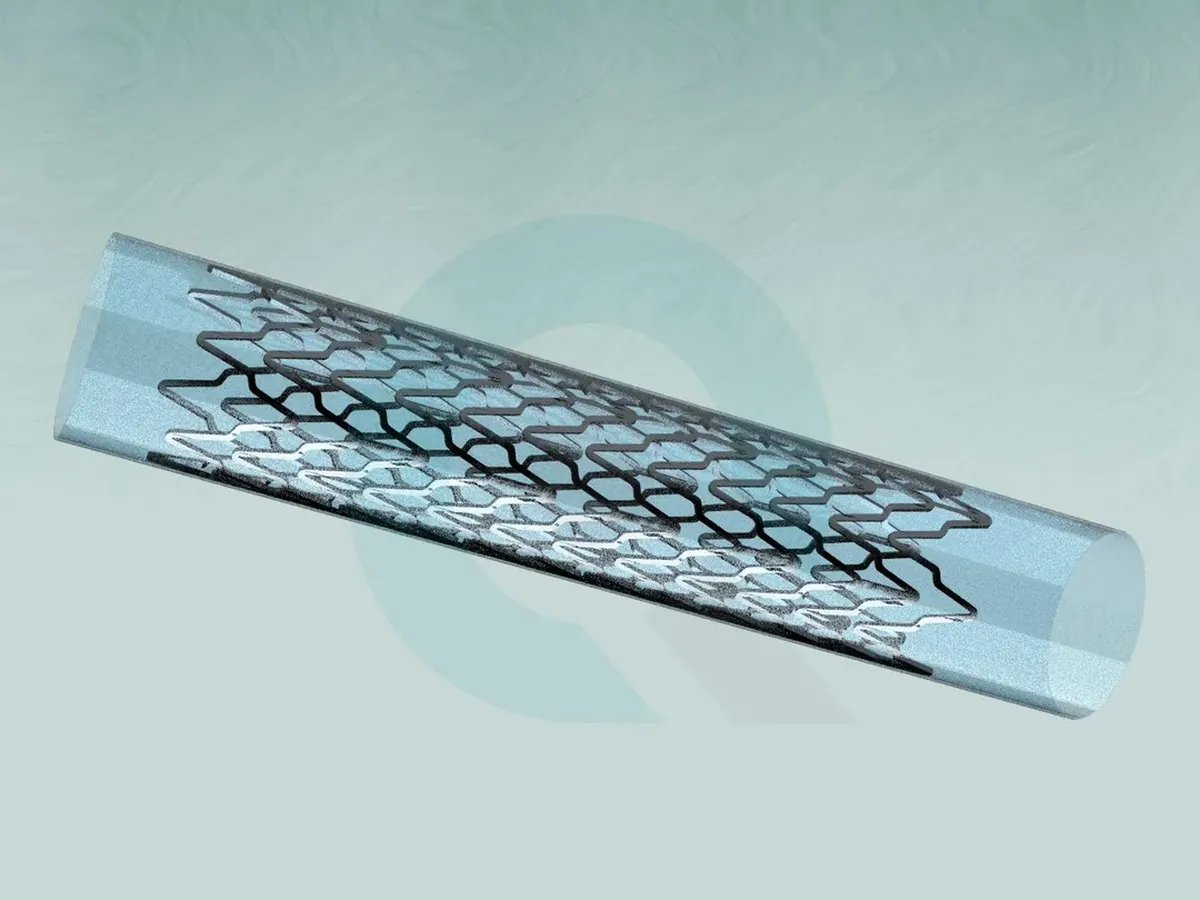
Stent - Fatigue and Durability Test
Fatigue and Durability Test - in vitro testing - of Stents evaluates the long-term structural integrity of the stent under cyclic loading.
Tests run for 380 million cycles in 37 °C tempered 0.9 % saline solution according ISO 25539-1, ISO 25539-2, ASTM F2477 or ASTM F3211.
Vena Cava Filters - Fatigue and Durability Test - ISO 25539-3
Fatigue and Durability Test - in vitro testing - of Vena Cava Filter evaluates the long-term structural integrity of the filter under cyclic compression.
Compression tests run for 80 million cycles in 37 °C tempered 0.9 % saline solution according ISO 25539-3 or ASTM F3211.
Biocompatibility - Nickel Ion Release
Our lab performs nickel ion release testing (nickel leaching) according to ASTM F3306 in accordance with Chapter III. C. 1.
Biocompatibility - Nickel ion release
FDA guidance document 1545, "Select Updates for Non-Clinical Engineering Tests and Recommended
Labeling for Intravascular Stents and Associated Delivery Systems Guidance for Industry and Food and
Drug Administration Staff" Document issued on: August 18, 2015.
We also perform other
corrosion tests and galvanic measurements.
Questmed Occluder Skills / Expertise
Download a presentation of questmed occluder testing with skills / expertise, presented at DIN workinggroup session 2021-06-18 - NA 027-05-06 AA "Herz- und Gefäßimplantate".Download "Questmed Occluder Skills / Expertise" as PDF.